HL Chemistry - 2¶
The course code for this page is SCH4UZ.
Thermal concepts¶
Definition
- A system consists of reactants and products being studied, often represented as a chemical equation.
- The surroundings/environment are all matter outside of the system capable of absorbing or releasing energy.
- Open systems allow energy and matter to move in and out of the system.
- Closed systems allow only energy to move in and out of the system.
- Isolated systems do not allow energy or matter to move in and out of the system. This is an ideal but unrealistic scenario.
Changes¶
As breaking bonds requires energy and forming bonds releases energy:
Definition
- An endothermic reaction overall requires energy.
- An exothermic reaction overall releases energy.
Physical changes such as state changes or dissolving substances may release or require energy depending on the energy of intermolecular bonds being broken and formed.
Example
- Ice melting requires energy to break the stronger bonds in a solid.
- Dissolving salt in water breaks the intermolecular bonds holding the salt together but regains it all by forming new bonds with the water.
Chemical changes all involve breaking old bonds to form new bonds. Depending on the energy required/released in breaking/forming those bonds, the reaction may end up endothermic or exothermic. Regardless, all reactions need a small initial activation energy to begin.
Info
Acid-base reactions are always exothermic.
Specific heat capacity¶
Please see SL Physics 1#3.1 - Thermal concepts for more information.
Enthalpy¶
Represented as in joules, enthalpy represents the total energy in a system. Absolute enthalpy is not measurable, so change in enthalpy () is often used instead. The magnitude of enthalpy change is dependent on the type of change and quantity of substance that is changing.
A negative indicates that energy has left the system and so is an exothermic reaction.
In a balanced chemical equation, change in enthalpy is written to the right after the product.
Example
Energy is required for the decomposition of water so its enthalpy is positive.
can also be included in a balanced thermochemical equation as a reactant or product instead of listed at the end. In this case, it is always positive and its sign determines whether it is a reactant or product.
Example
Using the same formula as in the previous example:
(Standard) Molar enthalpy of reaction¶
The molar enthalpy of reaction expresses the change in enthalpy when exactly one mole of the substance is involved in the reaction.
Example
The molar enthalpy of combustion (also known as the heat of combustion) of ethanol is , indicating that every one mole of ethanol combusted releases 1367 kilojoules of energy.
The standard molar enthalpy of reaction is the molar enthalpy of reaction when initial and final conditions of the reaction are at standard atmospheric temperature and pressure (SATP, 25°C @ 100 kPA). Therefore, the activation energy, energy released/required during the reaction, and energy released/required following the reaction to return to SATP are all included.
Warning
This includes energy required for some substances to change state, such as water vapour from combustion cooling to 25°C.
Energy profiles¶
Also known as reaction profiles, energy profiles are a visual representation of the change in chemical potential energy of the system.
- Absolute enthalpy () is placed on the y-axis while the reaction progress (time, sort of) is placed on the x-axis.
- A horizontal line representing the enthalpy before the change is placed at the beginning labelled with the reactants.
- A horizontal line representing the enthalpy after the change is placed at the end labelled with the products.
- The change in enthalpy is labelled with an arrow in the direction of the change with its value if known.
- A hump shows the reaction in progress (even exothermic reactions require some activation energy).
Bond enthalpies¶
, also known as bond association energies, the enthalpy of a bond type (e.g., ) is the energy required to break 1 mol of that bond type when the reactants and products are gaseous so energy is not lost from state changes. Compared of other methods of determining reaction enthalpy, this method is less accurate due to the other compounds affecting bond strength and thus enthalpy on a per-molecule basis.
The change in enthalpy of a reaction can be approximated by considering the bonds broken and formed:
Calorimetry¶
Definition
- A calorimeter measures changes in energy.
A basic calorimeter uses a lid and insulation to keep matter in and minimise energy changes with its surroundings. A thermometer is used to measure the temperature change of the water, and a stirrer is common to ensure accurate thermometer readings. The reactants are placed in water to react.
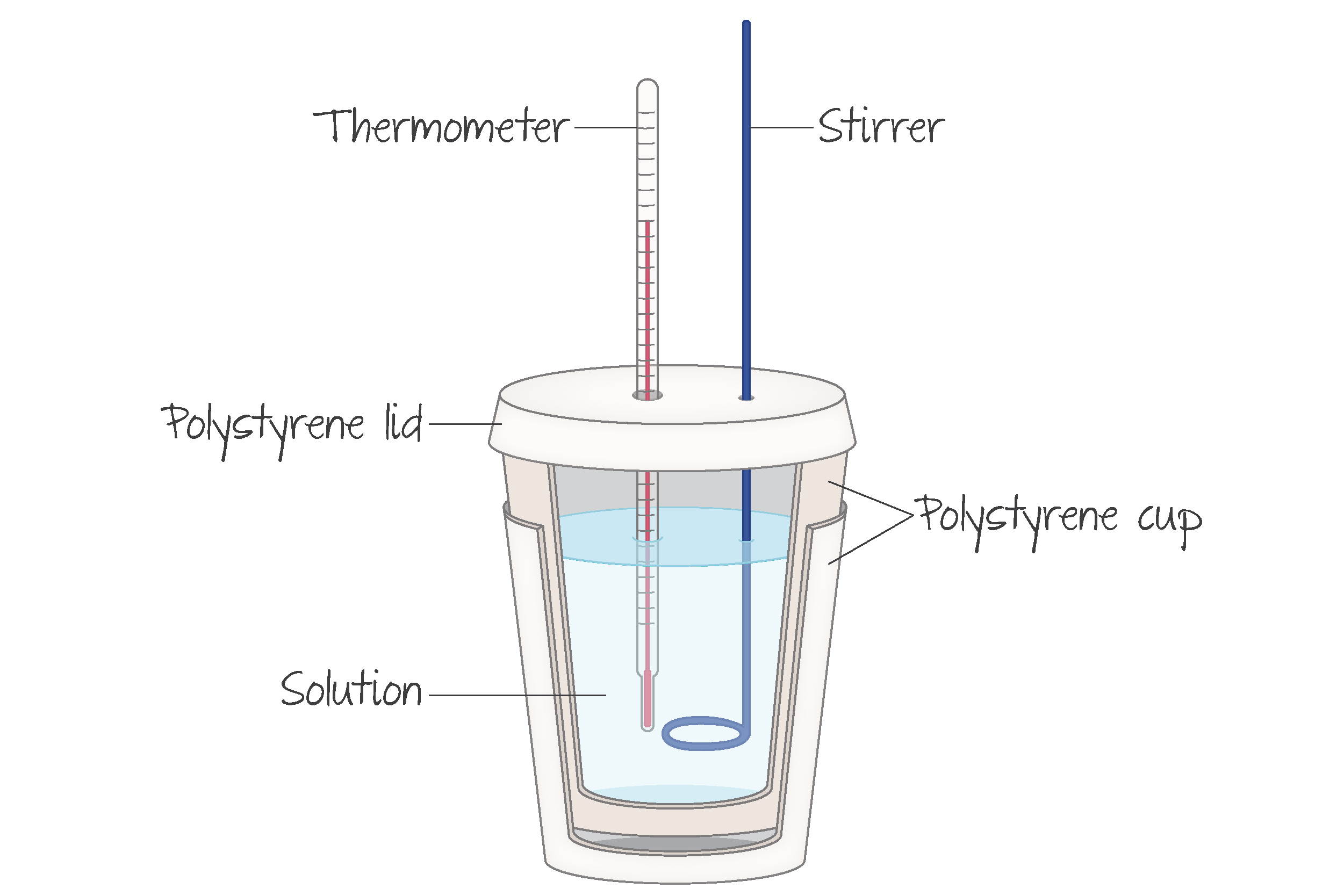 (Source: Kognity)
(Source: Kognity)
It is assumed that all the heat lost/gained by the reaction is gained/lost from the water.
In the event that reactants cannot be placed in water to react (e.g., combustion), a bomb calorimeter is used, which contains a metal sealed box submerged in the waterfilled with reactant and oxygen. A circuit leads into the box to start the reaction with a spark.
Warning
Assumptions in calorimetry:
- All energy released/absorbed from the system goes to/from the surroundings of the calorimeter (water). This usually needs to be corrected for in bomb calorimeters by measuring the heat capacity and mass of the metal box inside the calorimeter as well.
- No energy is transferred outside the calorimeter — the insulation should work properly.
- The calorimeter itself does not absorb or release energy — this is not a good assumption but can be compensated for.
- A dilute aqueous solution is assumed to have the same density and specific heat capacity as water — this assumption is best when the solute is diluted close to 1 mol/L.
Measuring calorimeters¶
Instead of recording the temperature of the calorimeter at any one point, a range of temperatures over time per trial should be plotted to obtain a curve. As calorimeters are not perfect and absorb/release energy, it will generate a graph that peaks and slowly returns to ambient temperature. To remedy this, the line returning the temperature to normal should be linearly regressed and extrapolated to the reaction start time to obtain a more accurate peak temperature.
Hess's law¶
Hess's law asserts that the change in enthalpy works like displacement - so long as the products and reactants are the same, any reaction with any number of intermediate steps will result in the same change in enthalpy.
Formation equations¶
A formation equation is a balanced chemical equation where exactly one mole of product and its reactants in elemental form are in their standard state — -gens are diatomic, phosphorus is , sulfur is , and at SATP (25°C, 100 kPa).
Info
Fractions are permitted as coefficients on the reactant side to get exactly one mole of product.
Example
The standard enthalpy of formation is the energy change from the formation of one mole of its substance from its elements in their standard states. It can be determined by subtracting the sum of the enthalpy of each element/compound on the reactant side and adding those on the product side.
Warning
It is assumed that there is no state change that would affect enthalpy when calculating standard enthalpy of formation.
Enthalpy cycles¶
Enthalpy cycles are a visual representation of Hess's law. It is used to show that the energy is the same from initial reactants to a product regardless of any intermediate steps.
Example
. Note that both arrows point to the intermediate product.
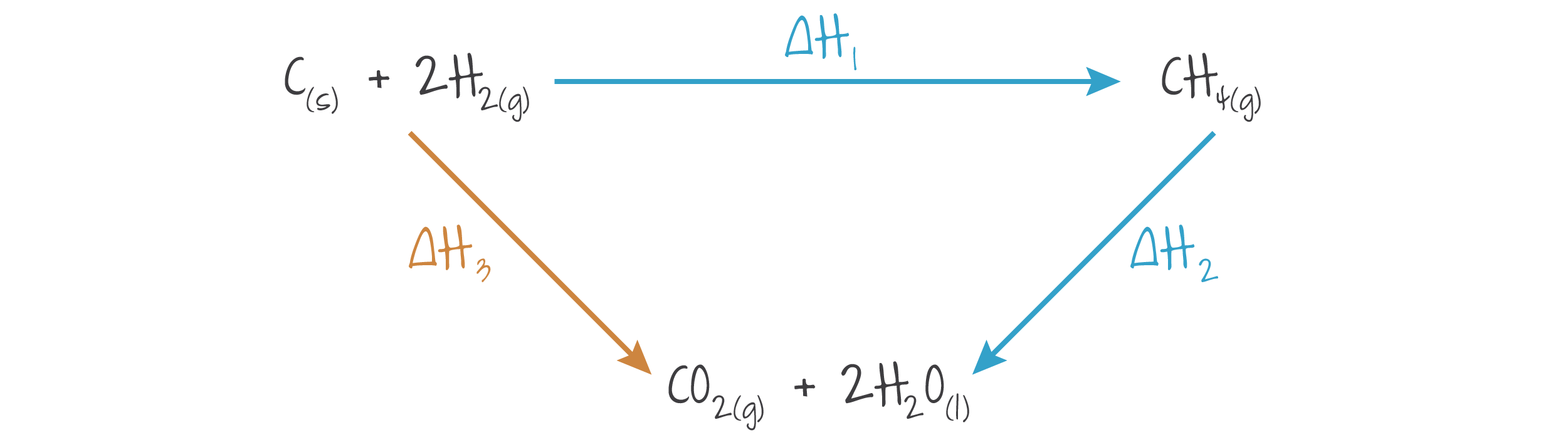 (Source: Kognity)
(Source: Kognity)
Born-Haber cycles¶
Definition
- The standard enthalpy of atomisation is the energy required to change 1 mol of an element at SATP in its standard state to 1 mol of atoms of that element in its gaseous state.
To form an ionic compound from elements in their standard states:
- the elements must be converted into gaseous atoms, (enthalpy of atomisation)
- the atoms must lose or gain electrons to form ions, (electron affinity/ionisation energy)
- and then the gaseous ions must bond to form an ionic compound.
The products should be listed on each level of a Born-Haber cycle, and relatively to-scale arrows should point in the direction of enthalpy change, where upwards increases enthalpy.
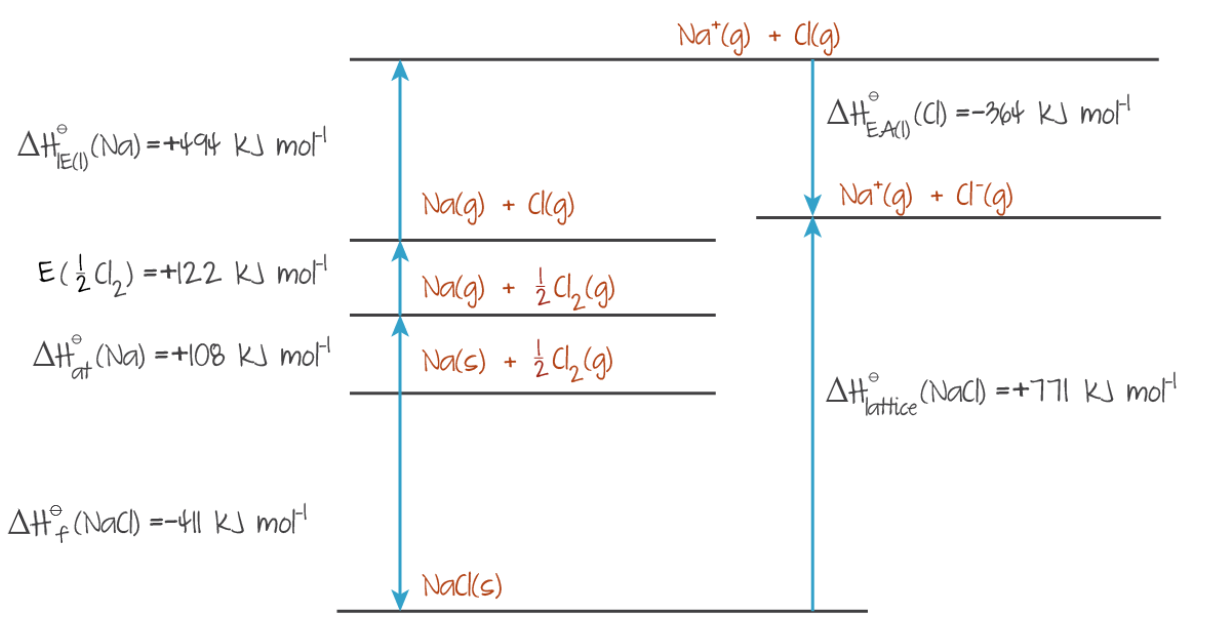 (Source: Kognity)
(Source: Kognity)
Second ionisation energy may increase the peak enthalpy after it has lowered from first ionisation energy. In this case, unlike the below figure, the first and second ionisation energies can be combined into a single arrow representing the sum of both.
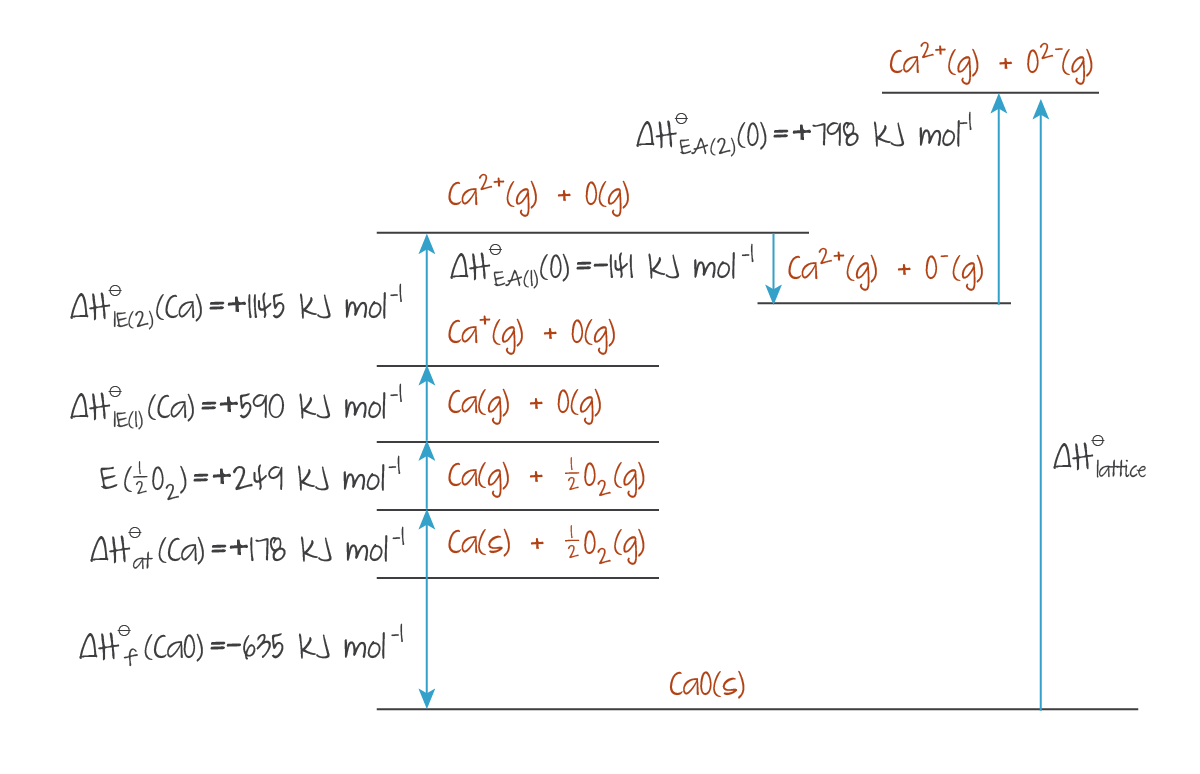 (Source: Kognity)
(Source: Kognity)
Lattice enthalpy¶
The lattice enthalpy of an ionic compound is the energy required to dissociate 1 mol of an ionic solid to its gaseous ions.
- It decreases as ionic radius increases due to greater distance and charge separation
- It increases as difference in charge increases because the greater charges are more strongly attracted
- The above only apply if the other (ionic radius/charge) is the same or similar
- Difference in charge has a much greater effect than ionic radius as it is multiplicative while the effect of increasing radius is additive
Enthalpy of solution and hydration¶
The enthalpy of hydration is the enthalpy change when 1 mol of a gaseous ion is dissolved in water to make an infinitely dilute solution such that it is unaffected by attraction or repulsion from other ions.
Example
The enthalpy of is the enthalpy of hydration of .
The enthalpy of solution is the enthalpy change when 1 mol of a substance dissolves in water. It is equal to the sum of the enthalpy of hydration and lattice enthalpy.
Example
The enthalpy change of is the enthalpy of solution of .
Entropy¶
Entropy, , is a measure of structural disorder in a system in . Absolute enthalpy is always positive, similar to enthalpy. An increase in disorder results in more entropy which results in a greater chance that a system will be in a certain state.
A reaction that increases entropy can continue even in the absence of extra energy, which results in endothermic reactions.
Reactions that would increase entropy are entropically favoured, so entropy will work to make it happen.
The following changes increase entropy:
- changes in state of one substance to a more disordered state, i.e., solid → liquid → gas,
- mixing particles of different types, e.g., solid to aqueous,
- increasing the number of moles of total gas or decreasing the number of moles of a solid,
- and increasing the number of moles of gas on the product side compared to the reactant side, which has the greatest effect.
Spontaneity¶
The spontaneity of a reaction is its tendency to continue without extra energy input after its initial activation energy.
Gibbs free energy or standard free energy (/, or ) is a measure of the sponetaneity of a chemical change. Spontaneous reactions must have a negative , while those that are positive will require more energy to continue.
Chemical kinetics¶
The rate of a reaction is the change of reactant to product per unit of time. The following are all viable methods of measuring rate of reaction:
- change in gas volume via gas collection,
- change in mass,
- change in light absorption,
- titration,
- and change in conductivity.
In an ideal gas, the kinetic energy of particles is spread in a Maxwell-Boltzmann distribution, where the total area under the curve is equal to the total number of particles in the sample.
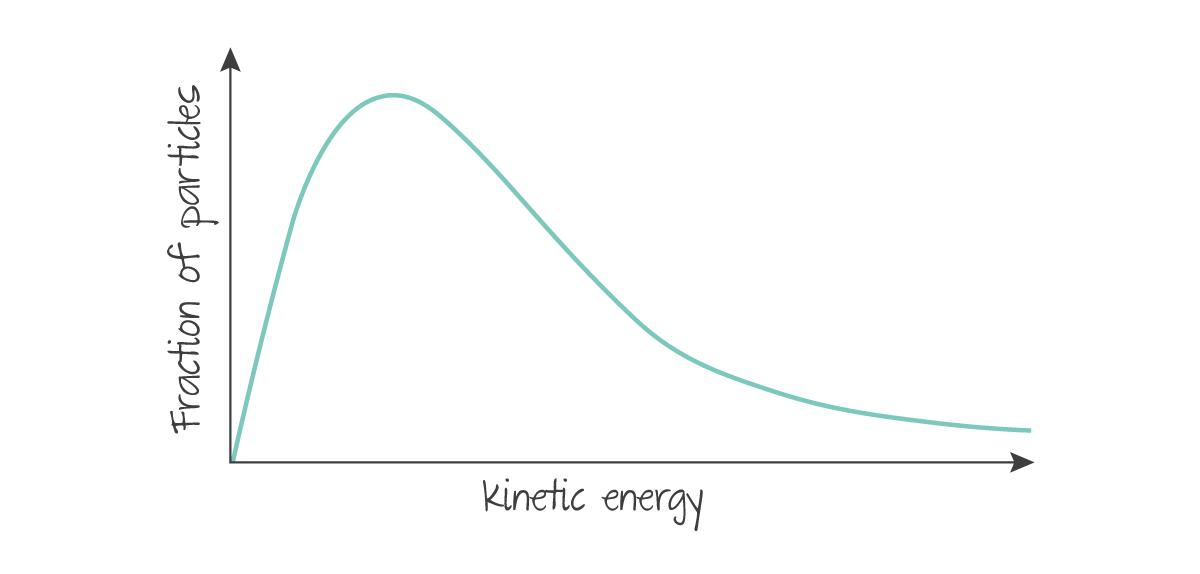 (Source: Kognity)
(Source: Kognity)
As temperature increases, the distribution's total area does not change but the overall spread moves to the right as more particles have higher kinetic energies.
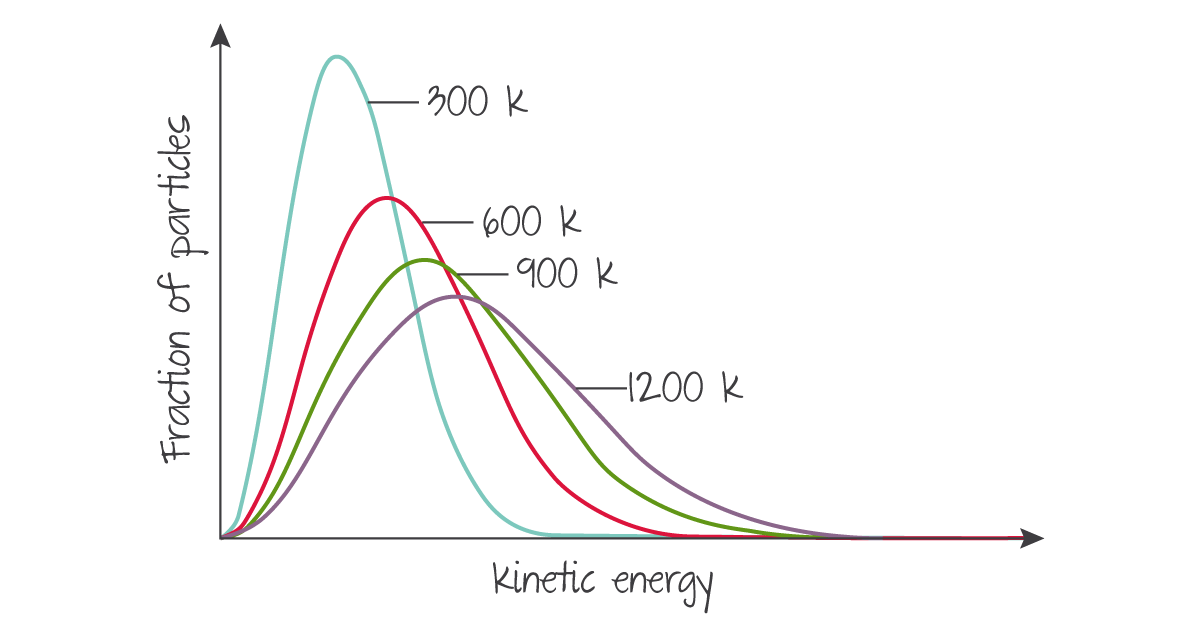 (Source: Kognity)
(Source: Kognity)
Collision theory¶
Collision theory states that for a chemical reaction to take place between two particles:
- they must collide,
- they must have proper collision geometry or collision orientation — similar to viruses bumping into cells, the "keys" must hit "locks" — in this case usually they must strike the bond,
- they must collide with enough energy to break the initial bond.
If all of these conditions are met, the collision is an effective collision — a collision that results in a chemical reaction.
The rate of a reaction increases with:
- the frequency of collisions,
- and the proportion of collisions that are effective collisions
Over time, the rate generally decreases because initially the highest concentration of reactants results in the highest collision frequency, which goes down as reactants are consumed. The proportion of effective collisions will also decrease as reactants also collide with product. Eventually, the reaction will stop or be so slow it appears to have stopped.
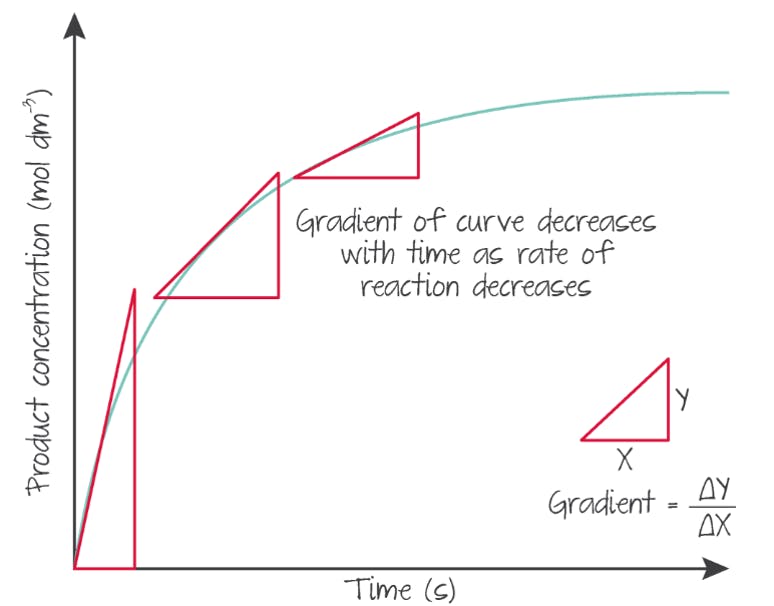 (Source: Kognity)
(Source: Kognity)
The following factors affect the rate of reaction:
- Surface area/particle size of a solid: as only particles on the surface of a solid can be collided with, smaller solid particles have greater surface area where more collisions can happen, leading to greater collision frequency.
- Concentration/pressure of reactant: A greater concentration leads to more reactant particles to collide in a given volume, increasing collision frequency.
- Temperature:
- Increasing temperature increases reactant particles' kinetic energy, increasing collision frequency,
- however it primarily increases the chance of particles having sufficient activation when they do collide, changing the proportion of effective collisions.
Activation energy¶
Because electron clouds repel reach other, without extra energy, particles would not get close enough to break bonds. This energy required for particles to become closer is known as the activation energy of a reaction. All chemical reactions have an activation energy requirement.
Catalysts¶
A catalyst is a substance that increases the rate of a reaction without being consumed. Not all reactions have catalysts, and increasing catalyst quantity does not necessarily always increase the rate of reaction.
Catalysts operate by reducing the activation energy needed by creating an alternative reaction pathway with a lower activation energy, so a larger proportion of particles are able to reach that lower energy requirement.
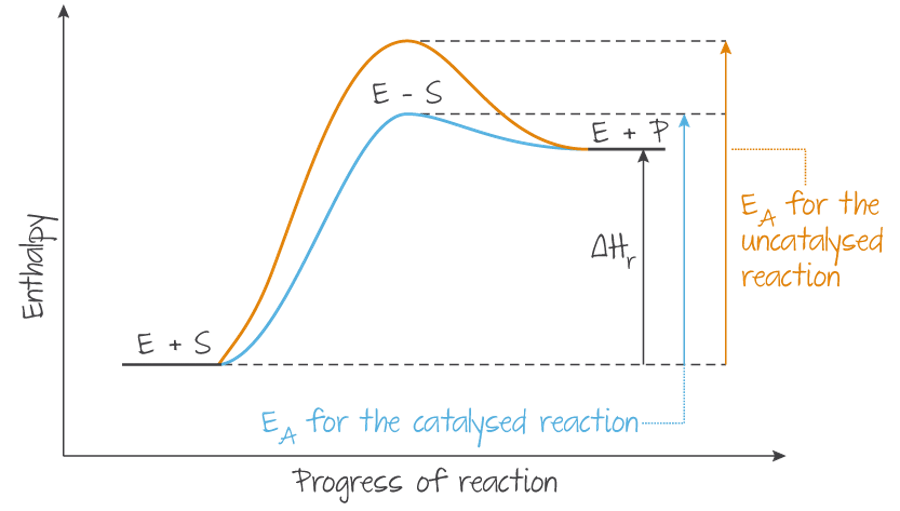 (Source: Kognity)
(Source: Kognity)
Visualised with a Maxwell-Boltzmann distribution:
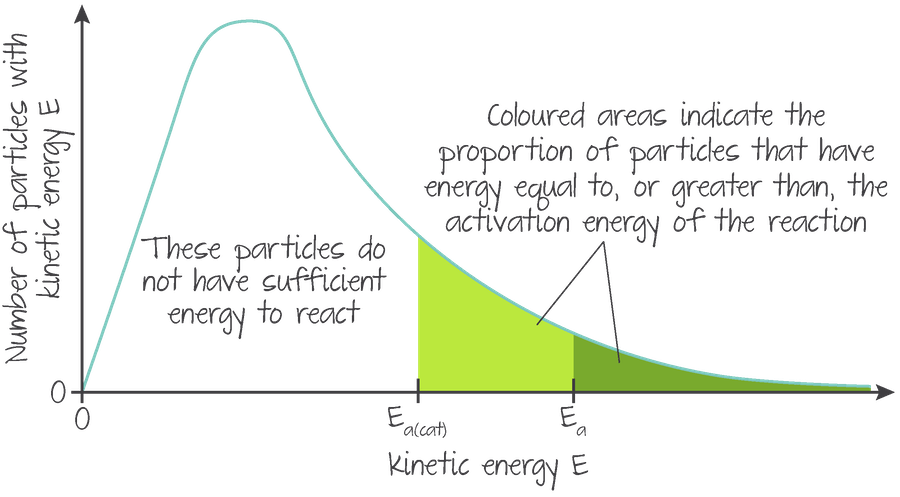 (Source: Kognity)
(Source: Kognity)
Catalysts can also improve the chances of correct collision geometry by encouraging certain orientations.
Rates of reaction¶
The law of mass action states that the rate of any reaction is directly proportional to the product of each reactant concentration. For a reaction of the form , the rate law holds that:
where is the rate constant, an empirically determined value that is only valid for one reaction at one temperature. Its units are equal to whatever balances out the equation — where is the order of reaction, it is equal to .
Warning
Solids and liquids have constant concentrations, so their factor is incorporated as part of and not included as a separate factor (e.g., not as ).
The individual order of reaction is the value of the exponent of a specific reactant in the rate law. It must be a real positive number.
Example
The individual order of the reaction with respect to is , and the order of reaction is .
To determine the individual order of reaction of a reactant, two identical experiments with equal quantities of the other reactants are needed. Where is the concentration of the reactant between the two trials, is the rate, and is the individual order of that reactant:
Example
For the following data, changing the concentration of by a factor of 3 causes a rate change by a factor of 9, therefore the individual order of is 2.
| Initial | Initial | Initial rate |
|---|---|---|
Integrated rate laws¶
Throughout the course of one trial of one reaction, a concentration-time graph can be used to find details about its rate. Where concentration is the concentration of the reactant in question over time:
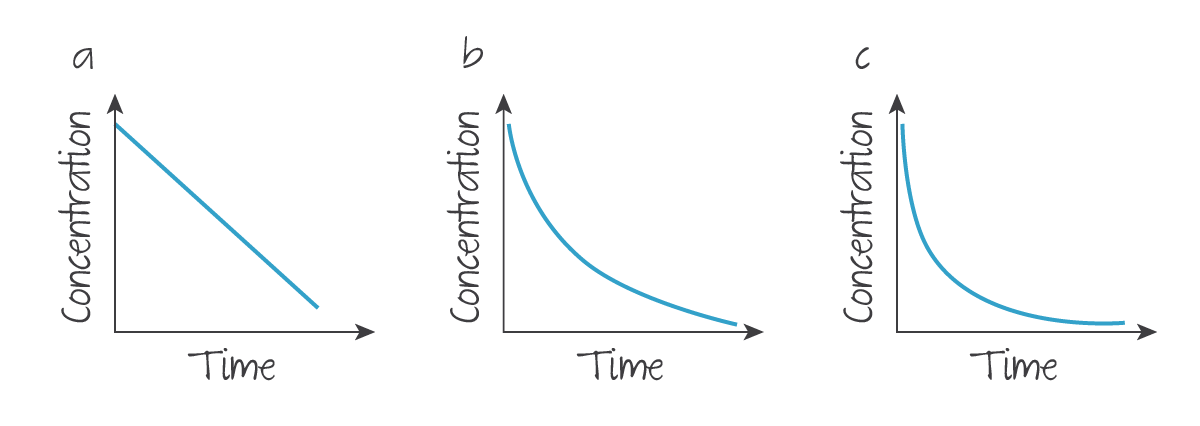 (Source: Kognity)
(Source: Kognity)
A reactant with an individual order of
- zero shows a negative linear line, and .
- one shows exponential decay, and of a graph of against time, which should be linear.
- two shows a deeper exponential decay, and of a graph of against time, which should be linear.
Additionally, a concentration-rate graph can be used.
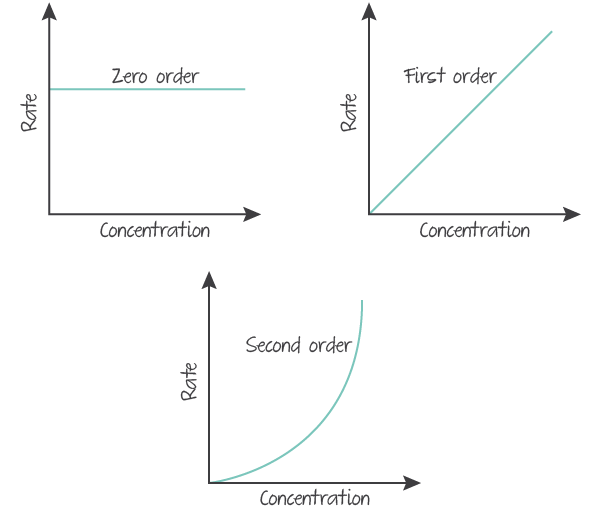 (Source: Kognity)
(Source: Kognity)
A reactant with an individual order of
- zero shows a horizontal line.
- one shows a positive linear line that passes through the origin.
- two shows the right side of a positive quadratic that passes through the origin.
Half-life¶
The half-life () of a reaction represents the time required for half of the sample to be used.
In the context of radiation, it is the time for half of the nuclei in a radioactive sample to decay.
In a zero-order reaction, each half-life is half of the previous.
In a first-order reaction, it is constant regardless of concentration, and can the concentration can be expressed with an equation, where is the concentration of a wanted substance, is the rate constant, and is the initial concentration.
In a second-order reaction, each half-life is double the previous.
Reaction mechanisms¶
Definition
- A multi-step reaction consists of more than one reaction as intermediate steps.
- An elementary step is the basic step of a multi-step reaction, usually involving one or two molecules but never more than three.
- A reactant is present initially but not at the end of a reaction unless in excess.
- A product is not present initially but appears at the end of a reaction.
- A catalyst is present both at the start and end of a reaction. It may be consumed and regenerated in intermediate steps.
- A reaction intermediate is not present at the start or end of a reaction as it is generated and consumed in the intermediate steps.
- The molecularity of a reaction represents the number of molecules that react in an elementary reaction from uni- to termolecular.
- An activated complex or transition state is the point where new bonds are being formed at the same time bonds are being broken.
A reaction involving any more than three particles will always take place under multiple steps because of the near-impossibility of such a perfect collision. Even reactions with three particles are often multi-step.
The reaction mechanism is the step-by-step sequence of all elementary steps of a reaction. An elementary step that is repeated consecutively should be surrounded with square brackets and a coefficient.
Example
Example
The reaction has a theoretical reaction mechanism of: is a reaction intermediate.
Multi-step reactions will have a rate-determining step, which is the slowest step and so is responsible for the rate law of the reaction, acting as a bottleneck. If reaction intermediates are present, the original reactants or catalysts that form that intermediate are still used in the rate law.
Example
The reaction has the following reaction mechanism: As normally for this reaction , because is a reaction intermediate, it is instead after substituting in the first step, ignoring product coefficients.
Often, the step with the highest activation energy is the slowest because of collision theory. Alternatively, the one with the least favourable collision geometry, such as if there are more particles that have to collide, may be the slowest.
If a reactant doesn't appear in the rate-limiting step (including via intermediates), changing its concentration will not affect the rate of reaction and so it will have an individual order of 0 in the final rate law.
A reaction mechanism is only plausible if:
- each elementary reaction has three or less reactant particles,
- the rate-determining step is consistent with the rate law provided, and
- the elementary steps add up to the overall equation.
Arrhenius equation¶
The Arrhenius equation relates the temperature to the rate of a reaction.
Where:
- is the rate constant,
- is the ideal gas constant,
- is the activation energy for the reaction,
- is the proportionality/Arrhenius constant for the reaction,
- and is Euler's number
Graphing against forms the linear relation:
where the slope of the graph is and the y-intercept is .
The number of moles of gas particles that are above the activation energy threshold is expressed in the second term of the equation: .
Equilibrium¶
Definition
- A reaction is at dynamic equilibrium if both the forward and reverse reaction continue at equal and constant rates, and there are no macroscopic changes such as temperature, colour, mass, or concentration.
A chemical equation at equilibrium is represented with two single-headed arrows, indicating that a reaction has proceeded to the point that concentrations are constant, and rates are equal and constant.
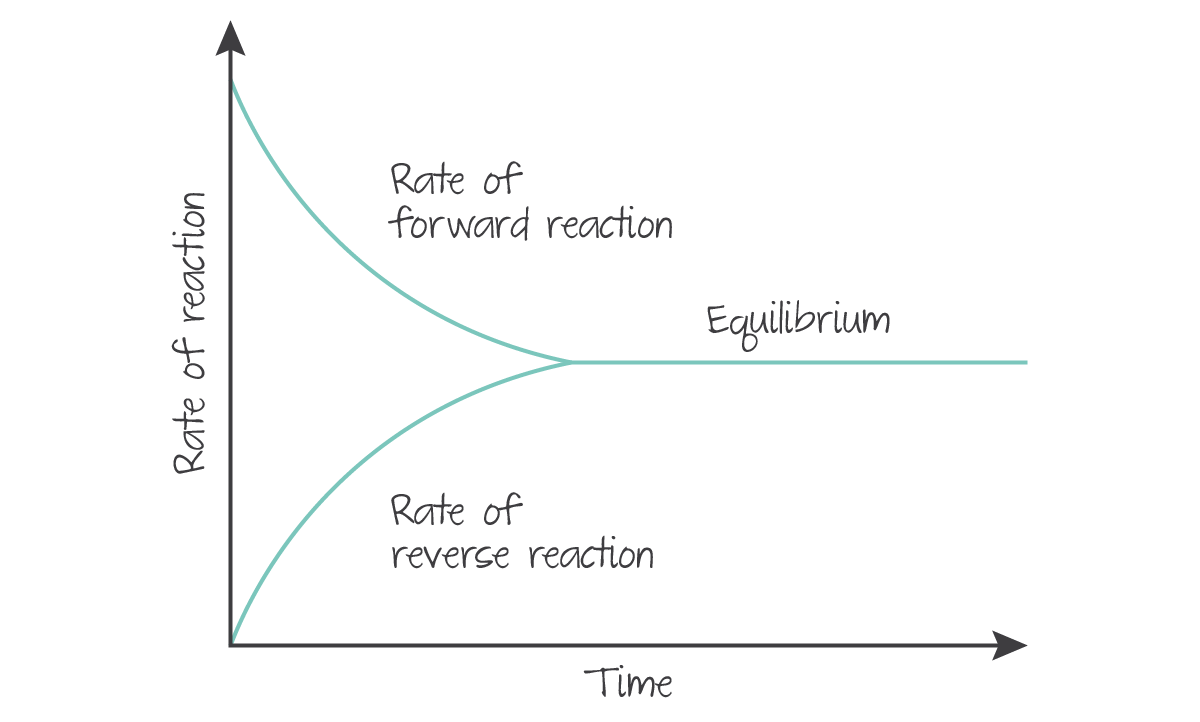 (Source: Kognity)
(Source: Kognity)
In order for a system to eventually tend to equilibrium, the system must:
- be closed, with constant concentrations of reactant and product,
- maintain a constant temperature, and
- maintain a constant pressure if the reactant or product is a gas.
For a given reaction, as long as the reactants and products are stoichiometrically matched, any combination will tend to the same equilibrium.
Example
The following initital concentrations for the reaction will all tend to the same equilibrium.
- 2 mol and 2 mol
- 2 mol
- 1 mol , 1 mol , and 1 mol
At equilibrium, the concentrations of the reactants and products must end up constant (but not necessarily equal).
Example
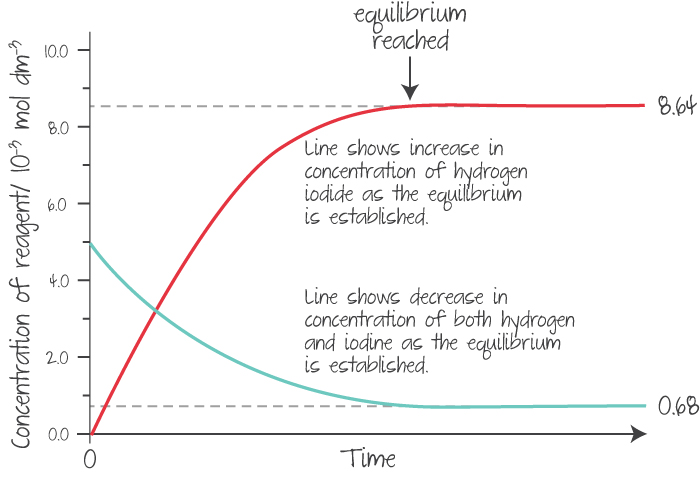 (Source: Kognity)
(Source: Kognity)
Phase equilibrium is when two or more states of exactly one pure substance are in dynamic equilibrium.
Warning
A solution or an aqueous compound cannot be in phase equilibrium because it is not a pure substance.
Example
Water constantly evaporates and condenses. Because the rate of evaporation is only dependent on the surface area of the water, the rate of condensation increases until the two are equal and constant at phase equilibrium.
A solubility equilibrium requires at least two substances — a solute and a solvent.
Equilibrium constant¶
Definition
- The position of equilibrium is the concentration of reactants and products at dynamic equilibrium.
The equilibrium constant or is related to the concentration of reactants and products in a given system at equilibrium at a given temperature. It is equal to the product of all products divided by the product of all reactants.
The units of varies similar to the rate constant so they are often omitted.
Warning
Only concentrations that change during the course of the reaction should appear in , so solids and liquid water should not be included.
If is greater than 1000, the reaction is product-favoured, meaning that there will be a greater concentration of products at equilibrium. If is less than 0.001, the reaction is reactant-favoured.
Contrary to the house of cards of lies told to you in lower grades, all reactions are equilibrium reactions, but some have s that are so large or small that they effectively occur to completion or don't occur at all.
ICE tables¶
An initial-change-equilibrium (ICE) table is used to work with equilibrium concentrations and only contains concentrations.
It consists of:
- the original concentrations of each compound in the "initial" row,
- the change in concentration in the form of a variable of each compound after one "iteration" of the reaction in the "change" row, and
- the end equilibrium concentration of each compound in the "equilibrium" row. The "initial" and "change" rows should sum to the "equilibrium" row.
Example
An ICE table with 1 mole each of and in of water that eventually ends up with an equilibrium concentration of will form the following ICE table.
| Initial | 0.50 | 0.50 | 0 |
| Change | |||
| Equilibrium | 0.11 | 0.50 |
When working with values involving , if the initial concentration of a chemical is much bigger than (), it is possible to assume that it will not change at all.
This assumption is valid if the impact of the calculated shift is less than 5%.
Example
If the equilibrium concenration is equal to , and the initial concentration is very big, assume that the equilibrium concentration is , removing the from the equation.
As long as is less than 5% of 0.250, the assumption is valid.
Info
In this course, when working with and ICE tables, only three things should be possible when solving for concentrations: you can get a perfect square, you can use the quadratic equation, or you can use the approximation rule.
Le Chatelier's principle¶
Le Chatelier's principle states that: If there is a change in a system at equilibrium, the position of equilibrium will readjust to minimise the effect of the change.
The changes that this principle affects — and therefore affect equilibrium — include changes in temperature, concentration, and pressure. These changes are assumed to occur instantaneously, which may result in sudden theoretical spikes in concentration-time graphs.
The initial rate of the change will start fast and then slow down, appearing as a sharp change instead into a curve in a concentration-time/reaction progress graph that never return to its original value.
Tip
Drawing horizontal dotted lines that represent the original position of equilibrium and vertical lines to represent the moment of system change makes it clearer to read.
Increasing the temperature of a system causes it to shift in favour of the endothermic side, and vice versa.
Of the three changes, this is the only one that would change as it changes the rate constants, which are temperature-specific (). Therefore, as temperature increases, also increases, and vice versa.
Example
If heat is added to a solution of KCl, more KCl will dissolve to minimise the change in temperature as it is an endothermic process.
Increasing the concentration of a reactant or product will cause the position to shift away from the increased side, and vice versa.
Example
If there is an instantaneous spike of to a system at equilibrium, it will be consumed along with to form , but not enough to return to its original value.
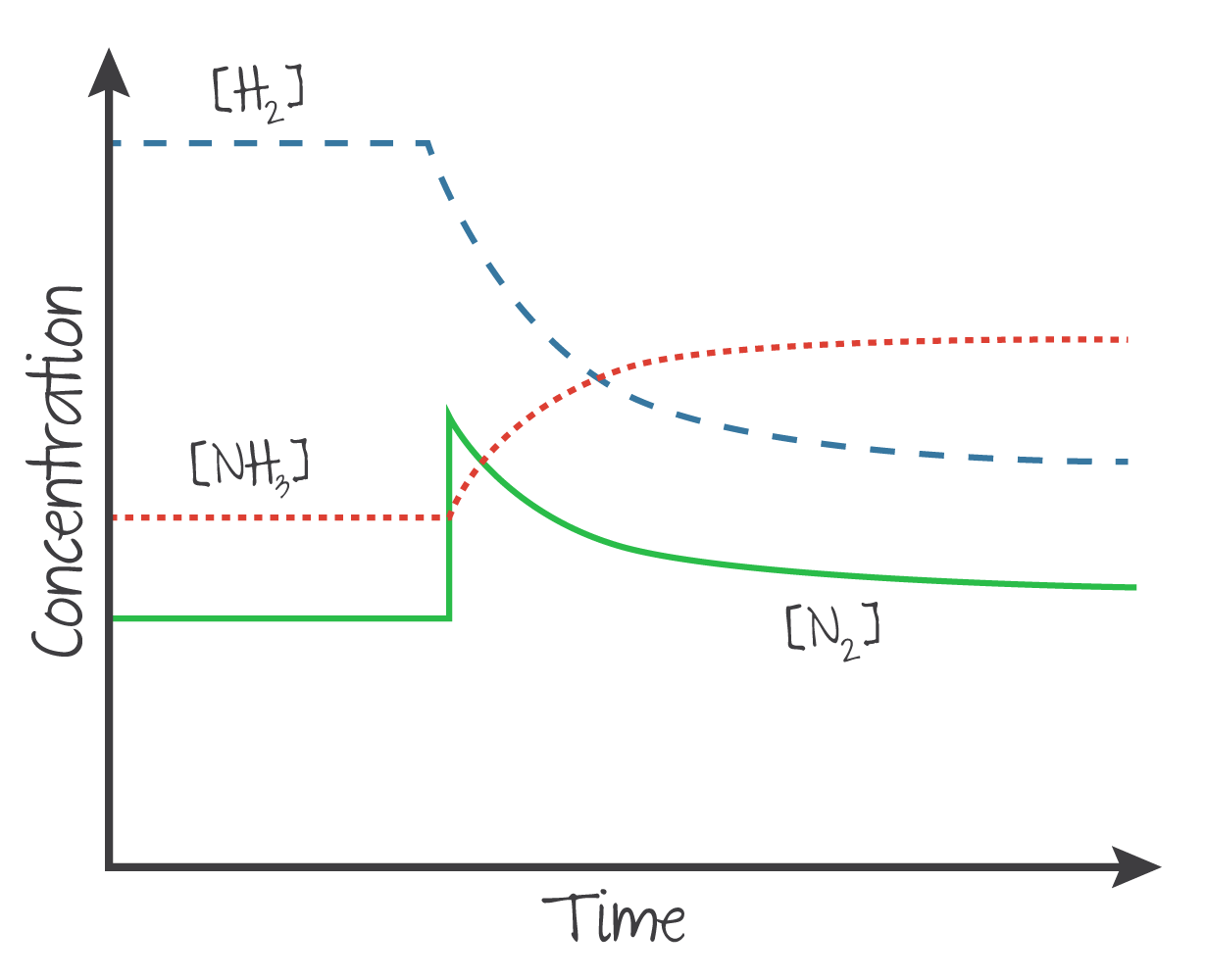 (Source: Kognity)
(Source: Kognity)
The same applies if instead is reduced.
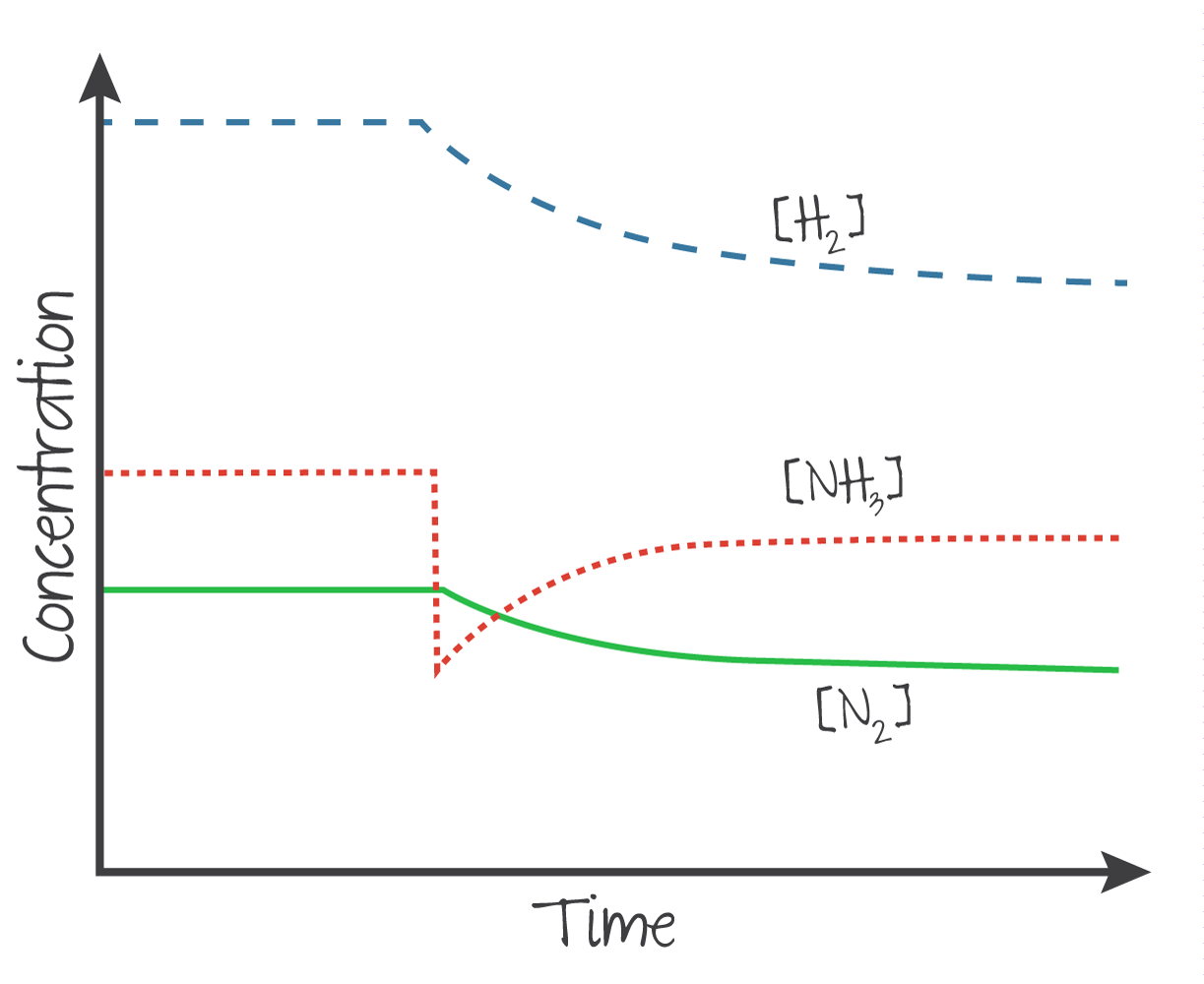 (Source: Kognity)
(Source: Kognity)
Increasing the pressure of a gas will cause the position to shift in whatever direction would decrease the total moles of gas.
Warning
Inert (uninvolved in a reaction) gases such as catalysts will not affect the position of equilibrium as it does not affect the partial pressure of the gas. In a similar vein, adding water to an aqueous solution will not cause any changes in equilibrium position.
Warning
If given a system not at equilibrium, if a change is made that would change the prior equilibrium, it should be assumed that the system reaches equilibrium before the change is made, regardless if it is specified.
Gibbs free energy 2¶
The value of Gibbs free energy changes as the reaction progresses, similar to enthalpy. At equilibrium, , so a reaction is a result of a system attempting to minimise Gibbs free energy.
Standard Gibbs free energy represents the Gibbs free energy of a chemical at standard state (1 mol/L for solutions, 100 kPa partial pressure for gases).
A negative indicates that the reaction will shift right to reach equilibrium as always decreases in magnitude as the reaction proceeds. It also means that the forward reaction is spontaneous while the backwards is not.
Reaction quotient¶
The reaction quotient () is a tool to compare the current state of a system to its equilibrium state.
At equilibrium, as they are the same equation, so the equilibrium will shift in whatever direction that would bring closer to
Example
If , there are more products than reactants than at equilibrium, so the reaction will shift to make more reactants.
Dynamic equilibrium¶
When is at a minimum, both sides of the reaction are equally spontaneous. Realistically, never reaches zero because entropy. TODO: wtf
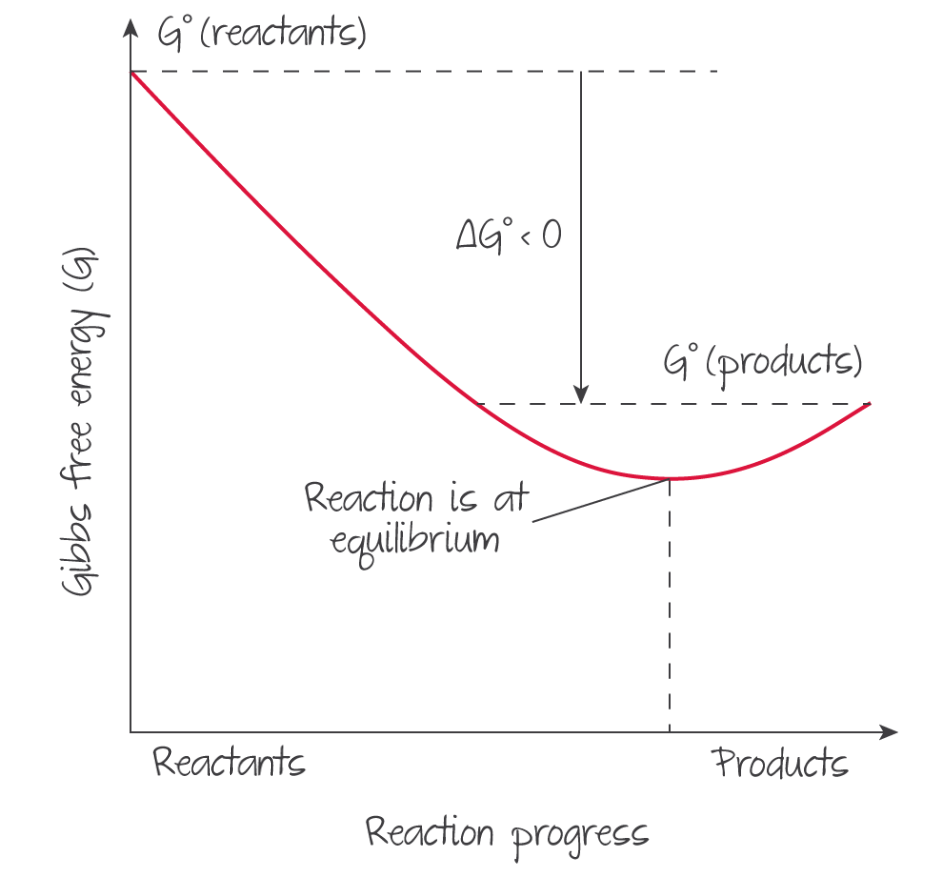 (Source: Kognity)
(Source: Kognity)
Where is the Gibbs free energy at a given point of the reaction, is the gas constant, is the current temperature, and is the reactant quotient:
Therefore, at equilibrium:
Acids and bases¶
Definition
- An amphoteric chemical may act as an acid or base depending on the situation.
- An amphiprotic chemical can either accept or donate depending on the situation.
- A monoprotic acid/base is one that can only accept/ionise one ion.
- An alkali/alkaline solution is an aqueous solution of a base, which may not necessarily be a basic solution.
An acid and base are any two corrosive chemicals that react to form water and a salt. They also dissociate/ionise (depending on theory) in water to form electrolytes that conduct electricity.
Acids:
- taste sour
- have a pH less than 7 in aqueous solutions at 25°C
- stain litmus paper red
- react with active metals to produce based on the activity series
- react with carbonates to form
Bases:
- taste bitter
- have a pH greater than 7 in aqueous solutions at 25°C
- feel slippery as they react with fats/oils to form soap
- stain litmus paper blue
- react with ammonium salts to product
Arrhenius theory¶
An acid dissociates in water to produce ions (protons).
A base dissociates in water to produce ions.
Bronsted-Lowry theory¶
The Bronsted-Lowry theory focuses on reactions with water and less the acid and base ions themselves, so they ionise instead of dissociate.
An acid is any compound that can donate/ionise a proton () to water to form a hydronium ion.
Info
In practice, the acid must contain a hydrogen atom attached by an easy-to-break bond (usually ), but any high electronegativity difference polar bond would work as well.
A base is any compound capable of accepting/removing a proton () from an acid.
Info
The proton usually comes from water. The base must be able to accept an ion to form a dative covalent bond, so they must contain lone pairs.
Polyprotic acids ionise their s one by one sequentially.
Example
Conjugate acids/bases¶
The result of a base obtaining a proton is a conjugate acid.
The result of an acid losing a proton is a conjugate base.
Example
In the reaction
is a base that becomes a conjugate acid while is an acid that becomes a conjugate base.
Louis theory¶
A Lewis acid is any species that accepts an electron pair to form a dative covalent bond.
A Lewis base is any species that donates an electron pair to form a dative covalent bond.
Strong/weak acids/bases¶
Strong acids/bases will completely dissociate/ionise in an aqueous solution. This means that the initial concentration of acid will be equal to the end concentration of .
All strong polyprotic acids initially have a one-way reaction then follow with equilibrium reactions.
Warning
Strength is a property of an acid and has nothing to do with its concentration.
Weak acids/bases will only partially dissociate/ionise in an aqueous solution, leaving behind most of the initial acid ( at equilibrium).
Warning
Measuring pH only returns , so it cannot be used to determine the concentration, identity, or strength of an acid.
All weak polyprotic acid reactions are equilibrium reactions.
Example
The following is a list of strong and weak acids:
| Strong acid | Weak acid | Strong base | Weak base |
|---|---|---|---|
| any | |||
| any | |||
| any | |||
To experimentally distinguish between a strong or weak acid/base, if their concentrations are equal, total ion concentration or concentration can be compared since the stronger acid ionises more.
Practically, this means comparing the rate of reaction with a metal or water or measuring conductivity as they reflect total ion count.
pH and pOH¶
This section will assume Bronsted-Lowry theory.
pH represents logarithmically on a scale from 0 to 14.
Warning
The number of sigfigs in pH is equal to the number of digits after the decimal place.
A solution is neutral (neither acidic nor basic) when . This happens to be at SATP. In pure water, this is true as a small number of water molecules react with each other.
In an equilibrium reaction between an acid and a base, , but water has a constant concentration, so the equilibrium of the two ions is represented with the water ionisation constant is used.
As temperature increases, increases, therefore changing the pH of neutrality, but this may not necessarily change the acidity of the solution as the ion concentration is still the same.
As pH increases, decreases, so must increase to keep constant and maintain equilibrium.
At 25°C, , so:
Acid/base dissociation¶
An equilibrium will be reached when a weak acid or base dissociates/ionises in water. The extent that the acid or base has dissociated/ionised can be quantified with percent dissociation/ionisation.
Note
When performing an approximation assumption in an ICE table, the assumption is also valid if the % ionisation is less than 5%.
The of acid ionisation/dissociation is known as , the acid dissociation constant.
The of base ionisation/dissociation is known as , the base dissociation constant.
Example
Warning
and only apply to acids and bases, respectively. Morphine, a base, does not have a , but its conjugate acid does.
At all temperatures:
Acid strength¶
A higher or indicates that the acid or base is stronger, increasing percent ionisation.
Strong acids/bases have an effectively infinite / in water, so they are all practically equally strong in water (this may not be true in other solvents).
As and are inversely correlated, an increase in leads to a decrease in .
The conjugate acid/base of a strong acid/base is effectively infinitely weak such that it does not affect pH at all.
Contrarily, the conjugate of a weak acid/base is measurably weak, strong enough to have / that affect the pH and act as an acid or base.
As indicates negative log, is inversely correlated with so that none of the variables can be directly compared without conversion.
Acidity of salt solutions¶
Definition
- A salt is an ionic compound that dissociates in water.
The pH of a salt solution depends on the combination of the acidity of each of its dissociated ions. Whichever is stronger pushes the acidity of a solution in its direction.
An ion originating from a strong acid/base is immeasurably weak and has no effect.
An ion originating from a weak acid/base is measurably weak and has an effect.
Example
For the salt NaCl:
As both and are strong, their conjugate acids/bases are both immeasurably weak, having no effect on the pH of the solution. Therefore, NaCl is a neutral salt.
If both dissociated ions have a measurable effect, the acidity depends on whichever is stronger via /.
Titration curves¶
Definition
- A titrant or standard is a solution with known properties that goes in the burette.
- A sample or analyte is a solution with potentially unknown properties that goes in the sample flask.
- The equivalence point of a titration is the point at which the solution is neutral .
A titration curve is generated in a titration where the pH of the solution is graphed against the volume of titrant added. Depending on the type or strength of the sample and titrant, different graphs can be generated.
Unlike the diagrams below, in a sketch, the following information is needed:
- the initial pH of the solution (the pH of the sample)
- the pH after a lot of titrant is added (assumed to be the pH of the titrant)
- the volume of titrant required to neutralise the sample ()
- the relative pH at the equivalence point (the relative pH of the salt solution)
The graph can be split into two halves: the acid half and the base half. In the following diagram, both the acid and base are strong, so their lines are identically shaped:
- the line starts at the initial pH, hugging the line, until,
- it sharply curves to the vertical, crossing the equivalence point, and
- continues vertically.
The same applies to the base but it instead ends at the final pH.
 (Source: Kognity)
(Source: Kognity)
In scenarios where the sample is a weak acid/base, instead:
- the line immediately briefly rapidly rises from the initial pH, then
- gradually increases, until
- a sudden curve to the vertical (but less sudden than a strong acid/base), and
- continues vertically.
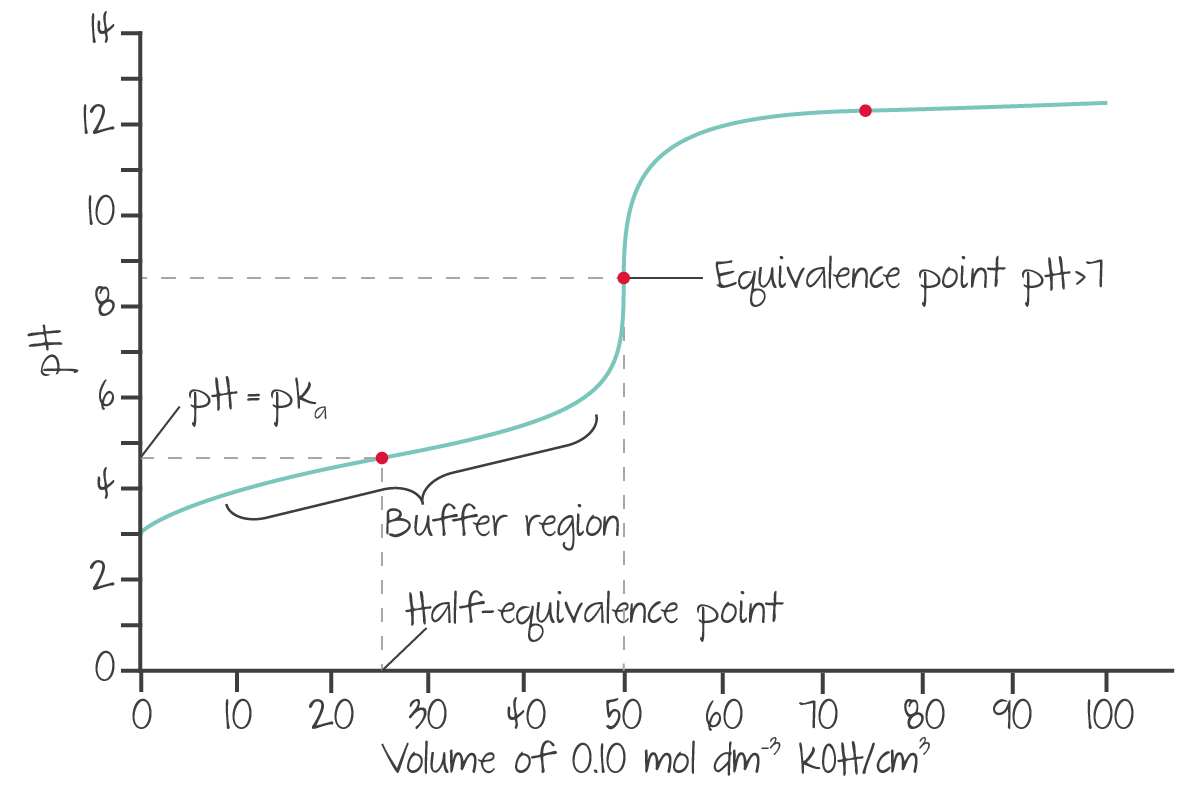 (Source: Kognity)
(Source: Kognity)
In scenarios where the titrant is a weak acid/base, it will take much more titrant to bring the pH of the sample to the level of the titrant. As such, the "brief rapid rise" is ignored and the line only gradually approaches but clearly does not reach the final pH.
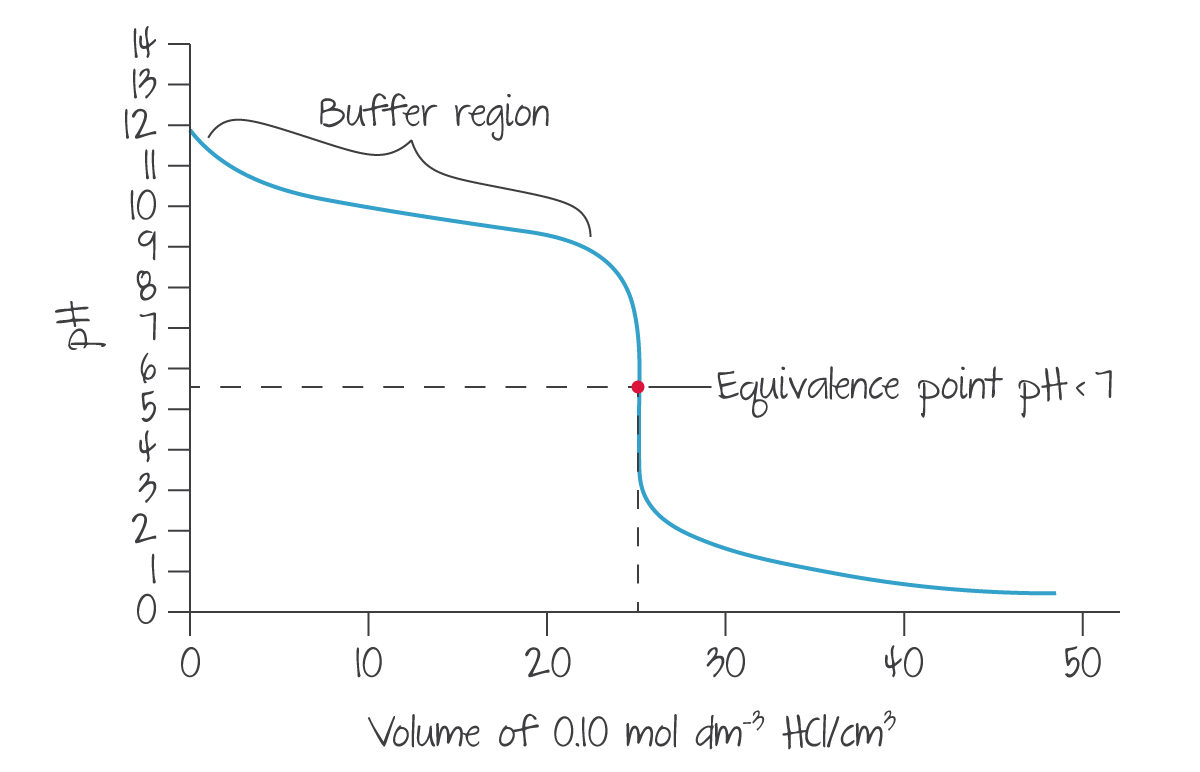 (Source: Kognity)
(Source: Kognity)
In scenarios where the sample is a polyprotic acid/base, as its ions dissociate sequentially, it can be treated as multiple consecutive titrations where the first sample is strong but any subsequent titrations are weak.
Each equivalent point volume after the first is a direct multiple of that first equivalent point volume.
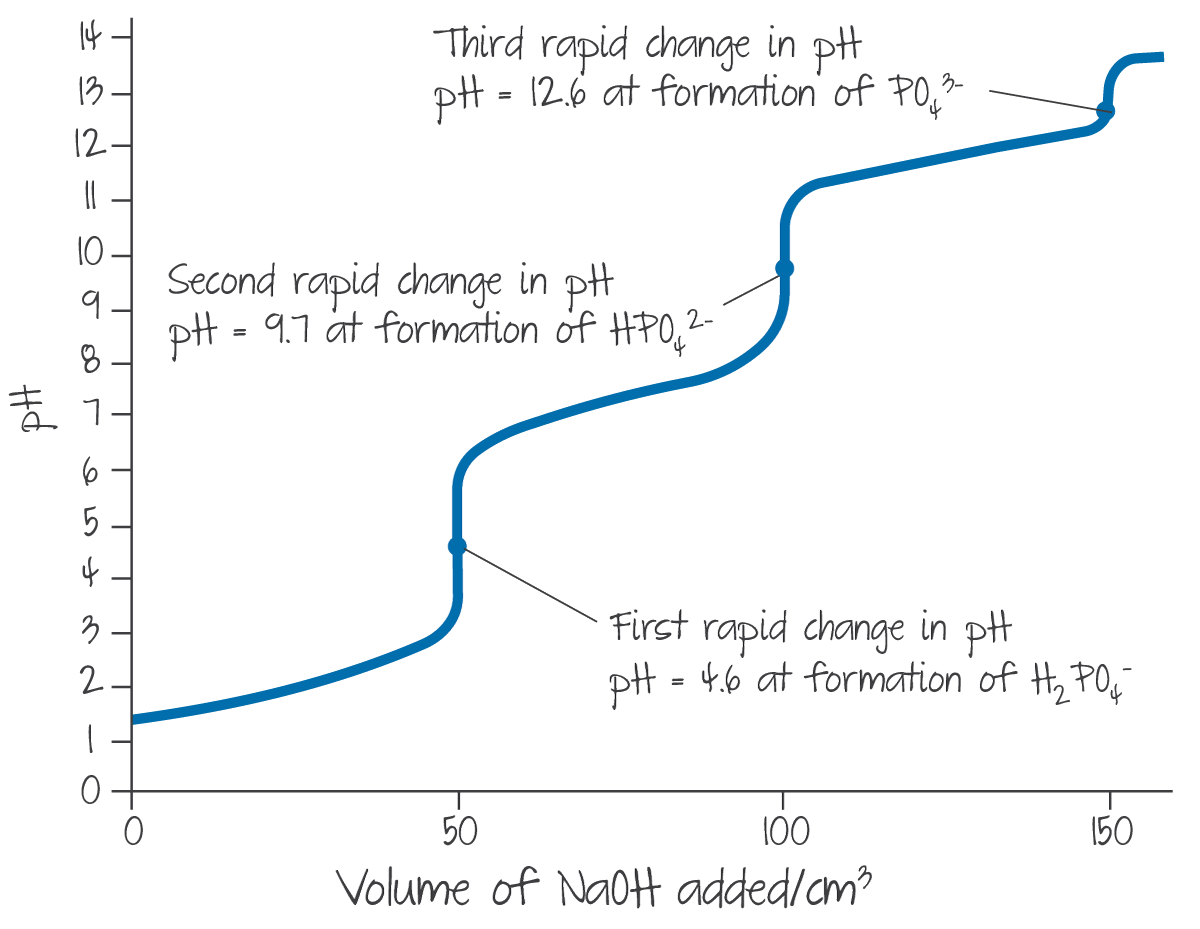 (Source: Kognity)
(Source: Kognity)
Titration curve analysis¶
The aha! equation, also known as the Henderson-Hasselbach equation, is derived from the equilibrium equation.
To graphically determine the of a sample given a titration curve, the pH at the volume at half of the equivalence point can be identified. At that point, .
Warning
- If a weak base is the sample, this will return the of the conjugate acid. The pH of the base can be determined by .
- If numbers are not given, drawing a line through the straight bits can give pH and equivalence volume values. However, none of these lines should be parallel to the axes.
- In titrations involving polyprotic compounds, as they are effectively multiple titrations, half of the equivalence point is actually half the distance between two equivalence points.
- This equation can also be used to determine the pH of a buffer between a salt/acid and acid/base as it can be assumed that there is no change to concentrations, but cannot be used to determine the initial pH between an acid/base and water.
pH indicators¶
A pH indicator is a weak acid/base that is at one colour in a certain pH range and another in another pH range. Where is the indicator, it will form an equilibrium with the hydrogen ions in the solution:
The indicator is protonated on the left and deprotonated on the right. The titrant can be viewed as an external stress on this equilibrium: if a base is added to an acid, the equilibrium will shift to the right to free up hydrogen ions, and vice versa.
If the difference in concentration of and is greater than approximately 10:1, the solution will appear to be the colour of the higher concentration, meaning that pH indicators will change colour at a pH in the range of their .
In choosing a good pH indicator, it must change colour in the vertical section of the titration curve to see the greatest effect, and it must be easily observable.
As the weak curve has less of a vertical section than a strong curve, it is best to pick an indicator that changes after the equivalence point, which will require the relative pH at the equivalence point.
The observability of an indicator depends on the colour it is changing to (or the direction the pH is changing). In general, humans are much better at noticing the appearance of red and blue.
A universal indicator is a mixture of different pH indicators to change colours multiple times over the pH range. In this case, the colour wheel can used to determine the colour that will be formed (e.g., blue + blue + yellow = green). The shade of the colour does not matter.
Buffers¶
Definition
- A buffer solution is one that can resist pH change when small quantities of a strong acid or base are added.
- An acidic buffer is one where an acid and extra of its conjugate base as a salt are present in the solution.
- A basic buffer is one where a base and extra of its conjugate acid as a salt are present in the solution.
- A protonated compound contains its proton.
- A deprotonated compound has lost its proton.
- The buffering capacity of a buffer is the quantity of strong titrant that can be added to the buffer without a significant change in pH.
The buffer region is the pH range of a weak acid/base before the equivalence point that requires a large volume of titrant for a gradual pH change. In this region, there is sufficient undissociated acid/base to replenish those neutralised via Le Chatelier's principle.
In the equilibrium between a weak acid and its component ions:
A buffer solution is created when excess is added (the salt of the conjugate base) such that the position of equilibrium is shifted to the left to the point that none of the original acid has dissociated such that and . It is used to maintain a certain pH in a solution.
When the titrant is added to an acidic buffer:
- if an acid is added, increases, shifting the position to the left. This can be done repeatedly because of the excess present to react with the protons.
- if a base is added, decreases as they react, shifting the position to the right. This can be done repeatedly because of the excess from the original shift to the left from the salt addition.
Example
To form the acetic acid/acetate buffer , if 1 mol/L is added to 0.1 mol/L :
The addition of will shift the position to the left, protonating it such that there will be 0.1 mol/L and 1 mol/L .
- If an acid is added, it will shift left and further react to form more , reducing the change in pH.
- If a base is added, it will shift right by reacting with hydrogen ions to reduce their concentration, releasing more to replenish the lost hydrogen ions, reducing the change in pH.
This naturally occurs without a buffer, but a buffer significantly increases the quantity of titrant that can be added before the pH changes rapidly.
The same applies to a basic buffer but in opposite directions. The salt of the conjugate acid is used instead.
Example
To form the ammonia/ammonium buffer , if 1 mol/L is added to 0.1 mol/L :
The addition of will shift the position to the left, deprotonating it such that there will be 0.1 mol/L and 1 mol/L .
- If an acid is added, it will shift right by reacting with hydroxide ions to reduce their concentration, releasing more to replenish the lost hydroxide ions, reducing the change in pH.
- If a base is added, it will shift left and further react to form more , reducing the change in pH.
To make an effective buffer, salt of the conjugate base/the conjugate acid is required to initially shift the position left. Adding more salt/acid increases the titrant that can be buffered.
A buffer only acts over a certain pH. In order for it to be effective, the ratio of to must be within 10x or 0.1x, although usually buffers are made with 90% excess salt/acid + 10% acid/base or vice versa. Using the aha! equation, this means that the range of a buffer is equal to , where is that of the acid/conjugate acid of the base.